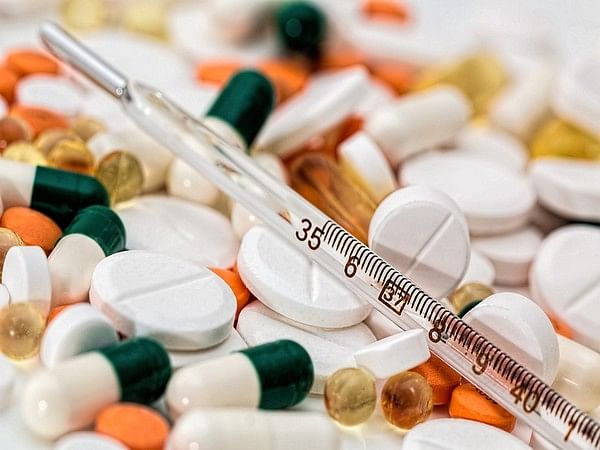
States and Union Territories have been urgently instructed to stop the sale and distribution of 35 fixed-dose combination drugs along with any other unapproved medicines.
“The approval of such unapproved FDC compromises patient safety and may lead to adverse of scientific validation. Upon issuance of show cause notices to the manufacturers, they have stated that these licenses were 19 granted by the respective Drug Licensing Authorities and have not violated any Rules. This has resulted in a lack of Uniform enforcement of the provision of NDCT Rules 2019 under the Drugs and Cosmetics Act, 1940, across the country,” the letter stated.
“Accordingly, all State/UT Drug Controllers are requested to ensure that the FDCs listed in the annexure–and any other unapproved FDCs–are not allowed to be manufactured, sold, or distributed in the country. They are also directed to investigate and take necessary action as per the provisions of the NDCT Rules, 2019,” it further added.
Fixed Dose Combinations, referred to as “cocktail” medicines. Such as painkillers, supplements, and Fertility combinations like Nefopam Hydrochloride 30mg+, Paracetamol 325mg tablets, Cefiximeip 200mg + Ofloxacin IP 200mg Lactic acid bacillus 60 million spores tablets, etc. (ANI)
This report is auto-generated from ANI news service. ThePrint holds no responsibility for its content.
var ytflag = 0;
var myListener = function() {
document.removeEventListener(‘mousemove’, myListener, false);
lazyloadmyframes();
};
document.addEventListener(‘mousemove’, myListener, false);
window.addEventListener(‘scroll’, function() {
if (ytflag == 0) {
lazyloadmyframes();
ytflag = 1;
}
});
function lazyloadmyframes() {
var ytv = document.getElementsByClassName(“klazyiframe”);
for (var i = 0; i < ytv.length; i++) {
ytv[i].src = ytv[i].getAttribute('data-src');
}
}